A BASIC DISCUSSION OF IONIC
CONTAMINATION TESTING
Ionic contamination testing has been an industry standard for at the last 12-14 years. Because of the formation of government/industry cleaning committees, cleaning evaluations to replace CFC’S, no-clean fluxes, heated ionic extract testing, and a host of new instrument manufacturers, there has been considerable confusion and misunderstanding about the test at all levels of use.Furthermore, there are many instances of our competitors misleading users, primarily because they themselves do not understand the basics of the test. Please use this handout to help clarify the issues associated with ionic extract testing, realizing that many questions today are still not yet addressed.
WHAT IS IONIC CONTAMINATION TESTING?
Ionic contaminants typically are water soluble, and are usually organic and inorganic acids and salts. Electronic hardware and components are frequently exposed to these contaminants in processing steps such as wave soldering, hot air leveling, plating, etching, and chemical cleaning. The test is performed by removing ionic residues from electronic parts with an ultra pure extract solution, and measuring conductivity or resistivity either during or at the end of the test.
The measured value is then compared to a standard. In this case, the standard is the measured conductivity or resistivity of sodium chloride in a pure 75% IPA/25% water solution. By determining the amount of NaCl that would be required to give this reading, the test result can be expressed either as a function of conductivity (or resistivity), or better, as an Equivalent amount of sodium chloride per area of sample. A sample test reading of 4.5 micro - grams NaCl/square inch means that the test ended with a conductivity / resistivity equivalent to what 4.5 micro-grams of sodium chloride would give - not that an actual 4.5 micro grams of sodium chloride was detected.
ORIGIN OF THE TEST
The first published method of contamination measurement extraction was by T.F. Egan of Bell Labs. The test originally used 100% D.I. water. Subsequent improvements such as the use of IPA/H20 mixtures to improve the removal of rosin residues were made at the Naval Avionics Center, Indianapolis, IN. This method required one to manually spray the test sample with the alcohol/water solution. Once the amount sprayed and collected was equal to 10 m1s per square inch of test sample surface area, the spraying was terminated and the resistivity was measured. This method became the original "manual method" per MIL-P-28809, with pass/fail limits requiring the extract solution to have a minimum of 2 megohms resistivity. Dr. Jack Brous of Alpha Metals independently developed an improved technique for testing, which was known as the "dynamic" method. Subsequent efforts to automate these test techniques resulted in two major types of test instrumentation - static and dynamic. The static test removes the contamination into a solution and makes a final measurement of resistivity or conductivity. This final resistivity is then compared to the equivalent resistivity of sodium chloride to give results in ugm NaCl/area. The extract solution is cleaned prior to the test.
The dynamic test method differs in that it is not an endpoint measurement, but rather a continuous measurement. After extract solution washes the part, instead of being re-pumped across the part to further remove contamination (like a static system), it is pumped thru the measurement electronics and then re-purified prior to being reintroduced. The extract solution can achieve much higher solubility and ionization characteristics than that of a static solution since it is ultra pure. Increments of contamination readings are "added up" or integrated over a period of time. The final amount of contamination is then compared to a standard sodium chloride reference to give an answer of ugm NaCl/area. The dynamic method is generally more accurate than a static method. Again, this is because the ionization of contaminants is greater in a pure solution as opposed to a dirtier or saturated solution found in a static test.
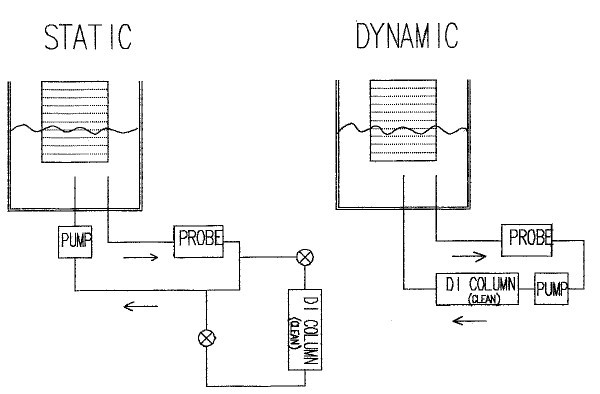
The diagrams previously shown indicate the basic differences in fluid flow. Although the test methods appear to have similarities to the casual observer, they are quite different in reality.
LINEARITY CHECK
A linearity check can be performed by adding progressive amounts of weak ionic contamination, and measuring the response. Ideally, the response will be linear with respect to the amount added.However, due to the differences in both solubility and the degree of ionization of the various species, both static and dynamic systems can differ.
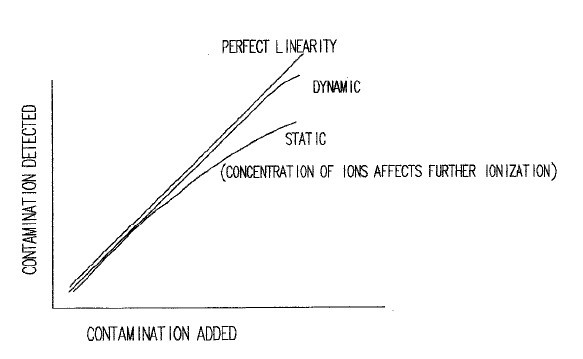
As we can see for weakly ionized materials, the response in the static system becomes more nonlinear as a typical test progresses. In the static test, the concentration of ions becomes greater and the degree of ionization decreases. In addition, weaker ions become more difficult to detect because of this high ion concentration. The dynamic test always operates with a very clean extract solution as ions are removed after detection. This results in a greater solubility and degree of ionization of contaminants, allowing even the weaker ions typically found in soldering fluxes to be detected. To illustrate this, consider a weakly ionized organic acid such as acetic acid.
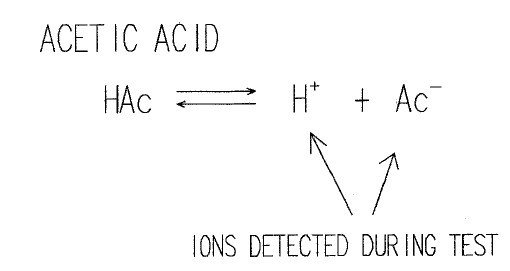
In an aqueous solution, the degree of ionization is limited. At higher concentrations, the degree of ionization is not in proportion to the concentration of undissociated acetic acid in solution. If a contaminant does not fully dissociate into its ionic states, some of it will go undetected. Furthermore the degree of ionization varies with the activator.
On a historical note, when the manual test as specified in MIL-P-28809 was developed, it was intended for rosin fluxes using amine hydrochloride types of activators. Generally speaking, these older flux formulations exhibited a more linear response with respect to concentration. Most water soluble fluxes are also dominated by highly ionizable amine salts and also exhibit good linearity characteristics. When testing the newer flux formulations of today (A’S, RMA's, water soluble’s), many have weak ionization characteristics, and would be more non-linear - or less accurately measured in a static system.
ISSUES TODAY
MIL-SPEC APPROVAL
Currently, only one manufacturer has been recently added to the MIL standards for an approved "alternative method". However, any user can perform the "manual Method" if desired. The manual method is based on 1960's assembly technology. Unfortunately, this method is obsolete when comparing it to the many significant assembly changes over the years, such as the requirements for higher density PWB and packaging designs. There have also been significant changes in the fabrication chemicals since the establishment of the manual test. For example, the earlier fluxes and activators of middle 1970's showed strong tendencies to ionize more completely than those of today. Current flux technology might contain several activator components, many of which would exhibit non-linear characteristics (if detected at all) when tested manually.
Fortunately, a joint industry/government task group is being assembled to outline, define, and implement a newer, more realistic qualification procedure for all instruments. Remember that the current manual method does not even account for improved efficiencies such as heated extract solutions.
PASS/FAIL LIMITS AND EQUIVALENCY FACTORS
Previously, there has been little activity within most government agencies (primarily DESC) as to not only establishing meaningful! pass/fail limits and equivalency factors, but as to understand why they were developed and what they actually mean. The equivalency factor is defined as a number indicating equivalence to the manual method (as described in "The Origin of the Test"). For example, the Omegameter equivalency factor was 1.39, and is 1.39 more sensitive than the manual method. Therefore, since the manual method established a pass/fail limit as 10 uGm/sq. in, the Omegameter's pass/fail limit was set as 1.39 times 10 uGm/sq. in. to give 13.9, or, 14.0 uGm /sq. in.
There still exist a large degree of uncertainty as to whether to put these equivalency factors into future specifications. There is a definite need to do so, for it makes little sense to test to a pass fail limit (as was once proposed) using instruments with variable degrees of accuracy and sensitivity. Currently, there is an effort involving both industry and government to re-evaluate the current testing methods, and to determine performance of different instruments from various manufacturers.
HEATED EXTRACT SOLUTIONS
Alpha Metals introduced the heated extract solution in 1987 since it was proven that rosin residues would not fully dissolve in room temperature solutions. The Omegameter 600SMD was designed to operate at a 115 degrees F as a minimum to enhance solubility of these rosin residues. Naturally, higher levels of contaminants were detected, and caused controversy because the pass/fail limits and equivalency factors were not updated to reflect the increased efficiency. At the present time, there are no known plans to incorporate extract solution temperature into military specifications. When comparing the effect of heating and agitating extract solution on the testing efficiency, heating proves to be the more significant contributor.
SPRAY VERSES IMMERSION
Considerable attention has been given to understanding the controversy of open spraying vs. submerged spraying. In the most severe cases of contamination, where "chunks" of residue are visible, open spraying may enhance removal. However the rate of dissolving these residues is primarily a function of extract solution temperature. In addition, solution turn over rate, volume, and agitation also affect the rate that contaminants dissolve. After dissolving, these contaminants must then ionize in order to be detected. As you can see, the key process steps are dissolving, and then ionization. If one is observing visible "chunks" of residues, it is recommended that more efforts be directed to improving the cleaning process as opposed to testing obviously dirty parts.
As discussed earlier, the degree of ionization is primarily affected by ion concentration, especially with the weaker acids - not whether the solution is being sprayed. Spraying in open air creates an additional problem not associated with submerged spraying, namely, CO2 absorption.
The Omegameter 600SMD has a very high turnover rate (7-8 gallons/min.) and enables the contamination to dissolve at a very rapid rate. The Ionograph 500M SMD turns over at a rate of 5-8 gallons/min., while continually introducing ultra pure solution back to the cell for optimum dissolving capabilities.
CARBON DIOXIDE ABSORPTION
As previously mentioned, once atmospheric carbon dioxide is exposed to the ultra clean extract solution, carbonic acid derivatives are formed. This weak acid ionizes and can thus be detected,causing measurement error. Any instrument that sprays in open air can be affected significantly by C02 absorption. Even the Omegameter 600 SMD is slightly affected by C02, although reasonably small.
It is easier to observe C02 absorption on a dynamic system as opposed to a static system. The static machines monitor at a lower resistivity, typically in the 60 Meg Ohm/cm region or less. It is at this range that resistivity is more insensitive - either to contamination or C02 absorption. However, when operating a dynamic instrument with a purer solution (100-500 Meg Ohm), the effect of open air spraying is dramatic, typically dropping the resistivity several hundred Meg Ohms in one minute. The Ionograph also subtracts out the "baseline" value which includes the initial C02 equilibrium value. In summary, it is impossible to spray in open air without compromising the results due to C02.
TESTING TIME
The testing time is a function of the speed of contaminant removal and dissolving, as well as ion concentration and its effect on ionization of the various ions. A dynamic test will usually run longer than a static test when testing highly contaminated assemblies because more contamination will be detected. Again, this is because more ions are generated due to minimal concentration effects on ionization. Several vendors have claimed that their instruments can perform a one to two minute test! This claim is simply irresponsible considering the variables that affect the test outcome, particularly the degree of ionization and its' relationship to ion concentration. Spraying the test sample may slightly increase the removal time of thick deposits of residues, but solution temperature, volume, and movement in capillary spaces determines how the contaminants will dissolve. If a user wants a quicker test without determining a true, final answer, then one should define a desired test time and do all testing for that length of time. Furthermore, most users running manual test times prefer an instrument that has much better accuracy as opposed to a so-called 1 to 2 minute tester. For example, when developing a fabrication process for Hi-Rel products, and where accuracy or reproducibility is critical, accuracy should not be compromised. Cleaning studies of flux residues are more meaningful with accurate instruments, as many newer formulations contain various combinations of strong and weak ions, of which many weak ions take longer to detect.
Comparing test time can be very misleading, as all instruments operate on similar extraction principles. The number one compromise for these "quick testers" is accuracy. If time of test is overly important, simply implement a manual test time of your choice for the product, using a longer test time to accurately qualify or set up the process.
IONICS AND ITS RELATIONSHIP TO SIR
There has been considerable questions from the industry in trying to correlate ionic contamination to surface insulation. After all the work done to date, There is virtually no data supporting any significant relationship using rosin fluxes. In the Phase 1 "Benchmark" program to eliminate CFC's, SIR was unable to differentiate between clean and dirty boards. When considering some of the water soluble fluxes, there usually is some relationship between SIR and flux residues, as these residues have a greater affinity for water - unlike rosin. However, at this time, these relationships have yet to be generated in a production worthy Manner. Data has been generated using certain classes of materials (lithium bromide for example), with the results being academic in nature, with little application for production.
SAFETY
There has been considerable interest as of late in safety issues. All of the Omegameters and Ionographs are intrinsically safe, but should be safety monitored similar to a wave solder machine or any equipment using alcohol based materials (such as fluxes and cleaners). At the present, the Ionograph 500 HD is the only instrument on the market built specifically to address safety issues. It was designed to meet or exceed Texas Instrument’s safety standards, but has not been submitted to Factory Mutual at this time. The older "Beckman" Ionographs were in fact submitted and approved by Factory Mutual Research for class I, Div. 2 rating. The current line of Alpha instruments are built with equal or better workmanship practices, although not FMR approved.
A possible solution to the flammability issue is to consider the use of an alternative solvent as a replacement for IPA. DPM is non-flammable, and performs well for the test. Studies to date indicate that a 60% DPM/40% H20 solution exhibit the best results. Alpha Metals is currently planning more extensive studies for use of this solvent in current instruments.
USER FRIENDLY OPERATION
The Alpha instruments have been designed to be user friendly, and have a long track record of operation in production facilities. Under normal circumstances, it takes approximately 10 - 20 seconds to key all information necessary to run a test. Repeat tests using the same conditions for other assemblies of the same type require even less time. |

